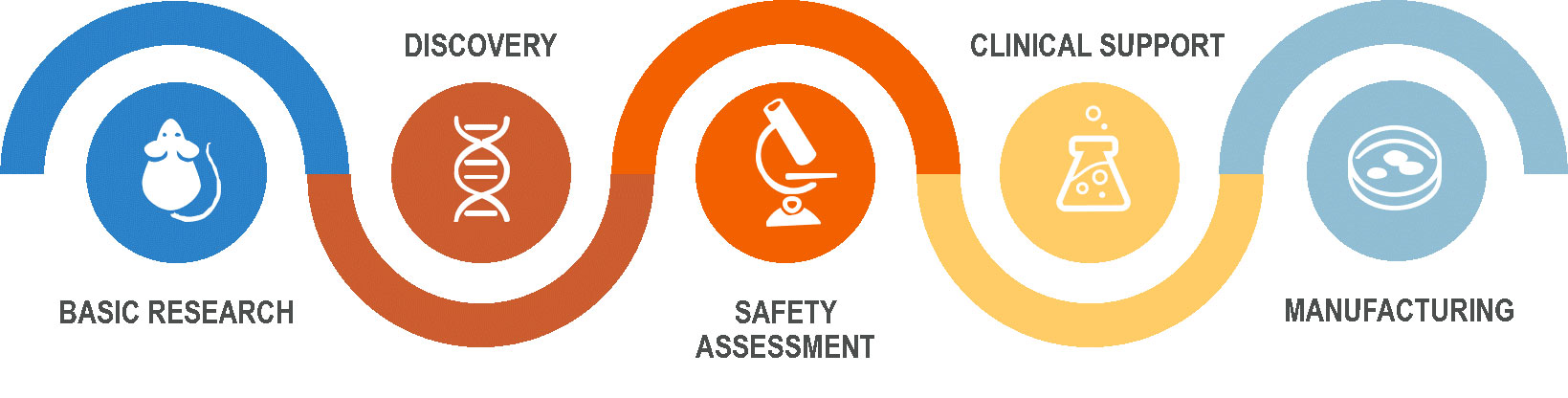
In a seminar held for the JGPT graduate and post-doctoral students, Dr. Learn explored the role of the CRO in the drug discovery, development and safety assessment industry. Contract Research Organizations (CRO) have become more of a development partner with pharmaceutical and consumer care industries when providing services and support in drug development. The students learned that the drug discovery process is long, complex, costly and is constantly evolving especially in the pre-in vivo space.

Dr. Learn also reminded the students that success can either be drug to market, or halting development because of unacceptable toxicity. Although society depends on cutting edge scientific development to make this process faster, better, smarter, and cheaper the main objective is to develop a safe drug and make a difference in people’s lives, not only patients, but everyone around them.
Dr. Learn started his career investigating both acute and chronic effects of ultraviolet radiation on the skin at Schering Plough HealthCare Products in the Advanced Products Research Group. He is now the Director of Photobiology and Cellular Therapeutic Safety at Charles River Laboratories, Horsham, Pennsylvania. Dr. Learn also took the time to answer any questions the students had regarding their career paths and explained to them the satisfying career opportunities that the CRO industry can provide.
